User Manual of Fillauer M074 Pediatric Formula Foot

To see other language options, visit fillauer.com.
Intended Use
The Pediatric Formula posterior-mounted, prosthetic foot is intended for use in lower extremity prostheses. The foot uses a long carbon pylon (Figure 1) to maximize energy storage and release during gait, similar to a “running blade”. The Formula foot is the ideal balance of flexibility and power. A first of its kind, the Pediatric Formula is a high-performance, customizable, pediatric foot that grows with the child. The long, lightweight carbon pylon provides critical energy return through the posterior attachment, while the compact shape of the ankle simplifies cosmetic finishing without hindering performance. It is also an exceptional walking foot for strong users and especially those with longer residual limbs and the height requirements that come with those limbs. The Pediatric Formula bridges the gap between everyday foot and sport specific prosthesis in a single device.
Indications
- Moderate to very high activity transtibial or transfemoral amputees
- Unilateral or bilateral patients
- Patients that would benefit from high energy return
- Patients that would benefit from low build height
- Patients weighing up 125 lbs. (57 kg)
Contraindications
- Clearance below 1.5 in. (3.8 cm)
- Patients weighing over (150 kg) 125 lbs. (57 kg)
The device is intended for single patient use only.
Performance Characteristics
- Patient weight: Up to (150 kg) 125 lbs. (57 kg)
- Foot weight: 12 oz. (340 g)
- Build height: 1.5 in. (3.8 cm)
- Durable; meets ISO-22675 standard.
- Primary Materials: Carbon composite, aluminum, and stainless steel
- Waterproof: The foot unit is waterproof to 1 meter. See additional information below
Storage and Handling
It is recommended that prosthetic feet be stored in a cool, clean, dry environment away from harsh chemicals (chlorine, acids, acetone, etc.).
Warnings and Precautions
![]() WARNING: For patient safety and device compatibility, only the appropriate Fillauer Posterior Mounting Bracket should be used with any Fillauer posterior mounted foot. Failure to use the appropriate bracket could cause the foot to break away from the socket and lead to falls or other patient injuries.
WARNING: For patient safety and device compatibility, only the appropriate Fillauer Posterior Mounting Bracket should be used with any Fillauer posterior mounted foot. Failure to use the appropriate bracket could cause the foot to break away from the socket and lead to falls or other patient injuries.
![]() CAUTION: Abnormal or improper environmental conditions will lead to malfunctioning and damage of the prosthesis and is not covered under the warranty of the device. This prosthetic/orthotic component must not be subjected to dust/debris, liquids other than fresh water, abrasives, vibration, activities which would damage the biological limb, or prolonged, extreme temperatures (< -5 °C or > 50 °C). Do not allow debris or liquids to remain in the prosthesis and its components during use. Rinse the foot with fresh water and dry immediately after exposure.
CAUTION: Abnormal or improper environmental conditions will lead to malfunctioning and damage of the prosthesis and is not covered under the warranty of the device. This prosthetic/orthotic component must not be subjected to dust/debris, liquids other than fresh water, abrasives, vibration, activities which would damage the biological limb, or prolonged, extreme temperatures (< -5 °C or > 50 °C). Do not allow debris or liquids to remain in the prosthesis and its components during use. Rinse the foot with fresh water and dry immediately after exposure.
![]() CAUTION: The foot unit is waterproof to 1 meter. However, if the foot is submerged, the foot and foot shell should be rinsed with fresh water and dried immediately to remove salt, chlorine, or debris. The foot shell and sock will experience significant deterioration if not allowed to fully dry before return to normal use and are not covered under warranty for this failure.
CAUTION: The foot unit is waterproof to 1 meter. However, if the foot is submerged, the foot and foot shell should be rinsed with fresh water and dried immediately to remove salt, chlorine, or debris. The foot shell and sock will experience significant deterioration if not allowed to fully dry before return to normal use and are not covered under warranty for this failure.
![]() NOTICE: The foot should be inspected by the clinician every six months for signs of abnormal wear and to assure that the attachment/alignment screws are secure.
NOTICE: The foot should be inspected by the clinician every six months for signs of abnormal wear and to assure that the attachment/alignment screws are secure.
![]() NOTICE: The foot stiffness is based on weight and activity level. Please provide accurate patient information so that the appropriate foot may be selected.
NOTICE: The foot stiffness is based on weight and activity level. Please provide accurate patient information so that the appropriate foot may be selected.
![]() NOTICE: Attachment, alignment, and delivery of the foot must be performed by or under the direct supervision of a qualified prosthetist. Any adjustment or modifications should be done by the clinician and not by the user.
NOTICE: Attachment, alignment, and delivery of the foot must be performed by or under the direct supervision of a qualified prosthetist. Any adjustment or modifications should be done by the clinician and not by the user.
![]() NOTICE: If any serious incidents occur in relation to the usage of the device, contact your Fillauer Representative and the appropriate authority in your country.
NOTICE: If any serious incidents occur in relation to the usage of the device, contact your Fillauer Representative and the appropriate authority in your country.
Installation
Attention: Deviating from the installation or modification instructions will void any product warranty and could lead to product failure and injury to the patient.
Pylon Versions
The Pediatric Formula foot is available in two versions. The standard version of the foot arrives with a 16 inch (41 cm) pylon that will need to be trimmed to length at the proximal end. The custom version arrives with a pre-trimmed proximal end and is ready to use with the included Pediatric Posterior Mounting Bracket (180-10-3000).
Pylon Modification — Standard Version
Trim the proximal end of the pylon just below the proximal, posterior brim of the socket. The pylon can be drilled and used with the Pediatric Posterior Mounting (PM) Bracket or laminated directly to the patient’s socket (see the Adult Formula Installation manual for direct mounting instructions). The pylon must be drilled as specified in the instructions for the PM bracket.
The distal end of the pylon must also be trimmed if it was purchased as an independent component. Use the guidelines printed on the forefoot to match with the desired foot plate length. Trim the foot using a band saw or cast saw and then sand to the indicated profile.
Pylon Modification — Custom Version
If the Custom version was purchased as part of a complete foot assembly, the pylon will be pre-cut. If purchased as a separate component, the forefoot of the pylon must be trimmed to match the desired foot plate length using the guidelines printed on the forefoot. The foot is best trimmed using a band saw or cast saw and then sanded to the indicated profile
Alignment (Specifications & Preparations Before Use)
Proximal Attachment
Permanent attachment of the foot may be achieved via direct lamination or through use of the Posterior Mounting Bracket. The Adjustable Alignment Bracket (PN 18- 10-5000) may be used for temporary, adjustable attachment during alignment. The Posterior Mounting Bracket (PN 180-10-3000), typically used for permanent attachment, may also be used in a temporary setting but only allows angular adjustments. See Posterior Mounting Bracket or Adjustable Alignment Bracket instructions for more information or request assistance from Fillauer for further instruction in this process.
Static Alignment—Sagittal Plane
Before aligning, the initial heel height should be established. The Pediatric Formula employs a 7° posterior lean (Figure 1) with a ¼ inch (6 mm) heel block to preload the anterior keel. When the patient is weight bearing, the socket bisection should settle to a vertical to slightly flexed position.

Figure 1
Transtibial Frontal Plane Alignment
A plum line from the bisection of the socket at the proximal brim in the frontal and sagittal plane should bisect the keel of the foot (Figure 2). The foot may be slightly inset 1–12 mm depending on the limb length. Most runners prefer a wider base of support with the foot slightly lateral to the distal bisection, 7–13 mm. The longitudinal axis of the foot will be externally rotated approximately 5–8° by aligning the medial border of the foot with the line of progression.

Figure 2
Transfemoral Static Bench Alignment
Alignment at the transfemoral level should be consistent with the instructions provided by the manufacturer of the prosthetic knee in use. Attachment to the prosthesis will be challenging with transfemoral amputees and the method of doing so is at the discretion of the treating clinician.
Dynamic Alignment
It is important to align the prosthesis so that the anterior keel is loaded sufficiently to provide dynamic response late in stance. Some bending of the carbon pylon is desirable for optimal performance and foot deflection may be more noticeable during dynamic alignment. Patient feedback during this process is essential. Use the Adjustable Alignment Bracket Kit for easier alignment of any Fillauer posterior mounted foot. If using the Posterior Mounting Bracket alone, adjustment of plantar/ dorsiflexion angles using the alignment wedges will help achieve a smooth transition from heel to toe and provide adjustment of transverse plane foot rotation.
- Check for smoothness of gait and ground contact throughout the stance phase of gait.
- If the heel rollover is delayed from heel strike to midstance, or the heel compression is too great, dorsiflexion of foot may correct this problem.
- If the heel rollover is too rapid from heel strike to midstance, or the heel is too hard, plantarflexion of the foot may solve this problem.
- If the rollover is too rapid from midstance to toe loading, increased plantarflexion may be required.
- If the rollover from midstance to toe loading is delayed, dorsiflexion may be indicated.
- Check to make sure pylon is vertical in the frontal plane at midstance. This angulation will be done by moving the bracket, so extra time spent in bench alignment to properly match the patient’s current angulation is advised.
Removing the Foot Plate
![]() CAUTION: Use a low heat setting to avoid overheating the composite material. Excessive heat may damage the foot.
CAUTION: Use a low heat setting to avoid overheating the composite material. Excessive heat may damage the foot.
Remove the old foot plate by using a heat gun to soften the epoxy glue which will aid removal of the screws. Apply heat to both proximal/distal surfaces around the screws by continuously moving the heat gun around the area with the hardware for 1 minute. The temperature of composite should not exceed 200 °F (93 °C). If unsure, it may be useful to periodically measure the temperature of the composite during this process. Remove the bolts and separate the pylon from the foot plate. If screws are difficult to remove, apply additional heat for another minute. Remove excess adhesive from the pylon with a rotary wire wheel or similar abrasive
Attaching the Foot Plate
All Pediatric Formula feet must have the foot plate bonded to the pylon. Once the pylon is trimmed (see above), attach the foot plate so that the split in the pylon matches the split in the foot plate. This can be done visually or by placing a tongue depressor or ruler into the splits to align them. Bond the foot plate using the provided epoxy adhesive or equivalent. Fabtech +PLUSeries adhesive is acceptable. Clean both bond surfaces with rubbing alcohol or acetone and let dry.
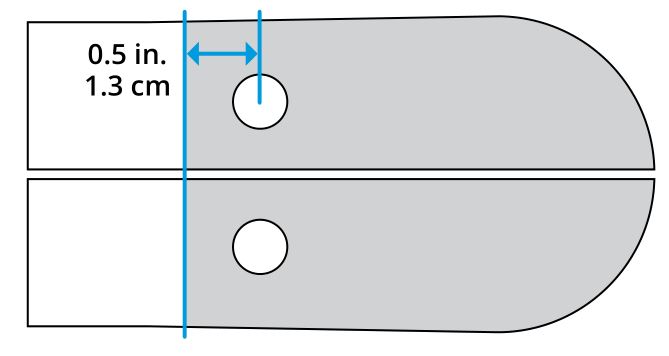
Figure 3
Apply adhesive to the underside of the pylon from the distal end to approximately 0.5 inch (1.3 cm) behind the bolt holes (Figure 3). Torque the bolts to 10–12 N·m with a 10 mm socket. Allow adhesive to fully cure before foot is used.
If a smooth stance phase of gait cannot be achieved, contact Fillauer for additional assistance.
Consumable Components: Foot Shell and Spectra® Sock
The Pediatric Formula uses a foot shell that can be purchased through Ossur (FSM Footcover FSM0 XX X, sizes 22–24 cm) or College Park (Enviroshell TP SXX XX, sizes 16–21 cm). See your Fillauer representative for details. Use care in the installation and removal of the foot shell to maintain its appearance and durability.
Always use the shell with an internal Spectra sock. Never use a sharp-edged tool such as a screwdriver to install or remove the foot shell.
Installation
- Slide the provided Spectra sock onto the foot from toe to heel, pulling excess material to the ankle so that it does not bunch under the heel or toe of the foot.
- Insert the forefoot into the foot shell as far as possible. Set the heel on a supportive surface with the toe up and push the shell onto the foot until the toe is in position.
- Rotate the foot side to side to allow the foot shell to slide onto the heel.
- Push the foot shell up onto the heel or, if necessary, insert a shoehorn into the foot shell and allow the heel to slide down a shoehorn into the heel lock. The heel must lock (Figure 3) in place for proper function and safety.
- The Spectra sock should be inspected and replaced if needed every 3-6 months by the prosthetist. The plantar surface of the foot should be inspected at this time and if there is excessive wear of the protective soling, it should be replaced.
- The foot shell should be inspected daily by the user and replaced by the clinician when tears or breaks are evident in the surface of the shell.
Foot Shell Selection
The Pediatric Formula foot plate comes trimmed for a sandal toe foot shell. Trim the posterior brim of the foot shell since it will most likely come into contact with the pylon as it exits. This can cause the foot shell to unclip from the foot.
Removal
- Place the foot on the bench so that the heel is hanging over the edge of the bench.
- Apply downward force to the top portion of the foot shell at the heel. The heel plate should pop out of the heel lock, allowing removal of the foot shell by hand.
- If the foot shell is too tight, a smooth-edged shoehorn may be used to disengage the heel lock.
Compatibility
Fillauer’s pediatric posterior mounting feet are appropriate for use with the pediatricsized Fillauer Posterior Mounting Bracket and Adjustable Alignment Bracket. A College Park or Ossur spectra sock and foot shell (not included) should be used with this device — the fit of other manufacturers shells cannot be guaranteed.
Disposal / Waste Handling
The product must be disposed of in accordance with applicable local laws and regulations. If the product has been exposed to bacteria or other infectious agents, it must be disposed of in accordance with applicable laws and regulations for the handling of contaminated material.
All metal components may be removed and recycled at the appropriate recycling facility.
Warranty
- 24 months from date of patient fitting
- Foot Shell (sold separately) – 6 months from date of patient fitting
User Instructions
The providing health care professional must review the following information directly with the user.
Care and Maintenance
![]() WARNING: If the foot performance changes or it begins to make noise, the patient should immediately contact his or her practitioner. These things may be as sign of a failure of the foot or other part of the prosthesis that could result in a fall or other serious injury.
WARNING: If the foot performance changes or it begins to make noise, the patient should immediately contact his or her practitioner. These things may be as sign of a failure of the foot or other part of the prosthesis that could result in a fall or other serious injury.
![]() CAUTION: Attachment, alignment, and delivery of the foot must be performed by or under the direct supervision of a qualified prosthetist.
CAUTION: Attachment, alignment, and delivery of the foot must be performed by or under the direct supervision of a qualified prosthetist.
Any adjustment or modifications should be done by the clinician and not by the user.
![]() CAUTION: The foot should be inspected by the clinician every six months for signs of abnormal wear and to assure that the attachment/alignment screws are secure.
CAUTION: The foot should be inspected by the clinician every six months for signs of abnormal wear and to assure that the attachment/alignment screws are secure.
![]() CAUTION: The foot is waterproof to 1 meter. However, if the foot is submerged, the foot and foot shell should be rinsed with fresh water and dried immediately to remove salt, chlorine, or debris.
CAUTION: The foot is waterproof to 1 meter. However, if the foot is submerged, the foot and foot shell should be rinsed with fresh water and dried immediately to remove salt, chlorine, or debris.
![]() CAUTION: The foot shell is designed to provide realistic appearance and maximum performance of Pediatric Formula.
CAUTION: The foot shell is designed to provide realistic appearance and maximum performance of Pediatric Formula.
![]() CAUTION: Patients should inspect the shell daily for signs of cracks or holes and for the presence of sand or other debris. If the foot shell shows signs of failure, it should be replaced as soon as possible to prevent damage to the carbon fiber and soling materials. If debris is present, the foot and shell should be rinsed and allowed too fully dry.
CAUTION: Patients should inspect the shell daily for signs of cracks or holes and for the presence of sand or other debris. If the foot shell shows signs of failure, it should be replaced as soon as possible to prevent damage to the carbon fiber and soling materials. If debris is present, the foot and shell should be rinsed and allowed too fully dry.
![]() CAUTION: The life of the foot shell will depend on level of activity and degree to which it is protected from wear and damage with socks and shoes. Socks and shoes should be worn at all times and should be allowed to dry fully after exposure to water to prevent damage to the shell.
CAUTION: The life of the foot shell will depend on level of activity and degree to which it is protected from wear and damage with socks and shoes. Socks and shoes should be worn at all times and should be allowed to dry fully after exposure to water to prevent damage to the shell.
![]() CAUTION: The foot shell may also be cleaned soft cloth and a soap and water solution or with rubbing alcohol (70%). Do not use acetone. It will damage the foot shell.
CAUTION: The foot shell may also be cleaned soft cloth and a soap and water solution or with rubbing alcohol (70%). Do not use acetone. It will damage the foot shell.
Serious Incidents
In the unlikely event of a failure resulting in a fall and/or injury, seek immediate medical help and contact your prosthetist at the earliest possible convenience.

Fillauer LLC
2710 Amnicola Highway
Chattanooga, TN 37406
423.624.0946
![]() 3938 S. 300 W.
3938 S. 300 W.
Salt Lake City, UT 84107
801.281.9964
![]() Fillauer Europe
Fillauer Europe
Kung Hans väg 2
192 68 Sollentuna, Sweden
+46 (0)8 505 332 00
![]() © 2021 Fillauer LLC
© 2021 Fillauer LLC
M074/04-10-18/10-04-21/Rev.1

