Forage Evolution Forager-Fast Plasmid Subcloning Kit User Manual

Product Manual
The Forager-Fast Plasmid Subcloning Kit contains all the necessary components to start using the next generation workhorse for plasmid subcloning and propagation. The host strain (Forager-1) is a variant of the fastest growing organism known, Vibrio natriegens (V-nat), supercharged with Active DNA Foraging to make plasmid transformations easier than ever. With a doubling time <10 minutes under ideal conditions and single incubation transformations, Forager-1 is the fastest and easiest way to transform and propagate plasmids.
Table of Contents
Kit Overview
Forager-Fast is a plasmid subcloning system using an engineered V. natriegens strain (Forager-1) with optimized transformation and growth kinetics. The kit is generally compatible with E. coli based workflows and supports a range of plasmid constructs. Although the transformation efficiency is less than other commercially available chemically competent cells, we’ve found that it is sufficient for many cloning workflows.

Kit Components
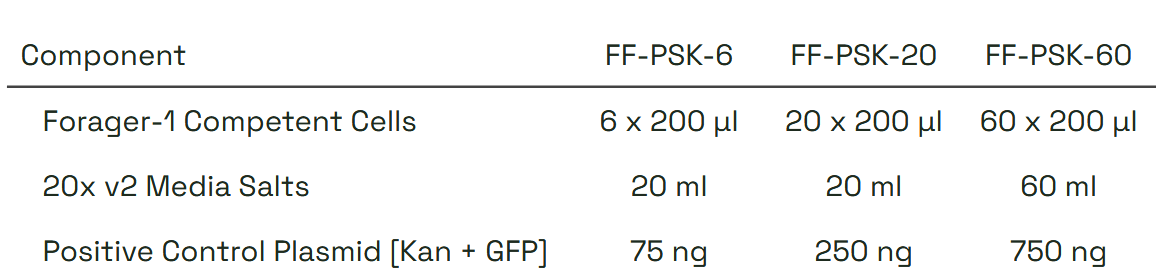
Storage & Handling
- Store competent cells at −80°C, avoid freeze/thaw cycles.
- Store 20x v2 Media Salts at room temperature, heat gently (~55°C) if precipitation occurs.
- Store the Positive Control Plasmid at or below -20°C.
- Thaw frozen components at room temperature.
- Competent Forager-1 cells are stable at room temperature for at least several hours, and remain competent even after vortexing.
- Use sterile, nuclease-free tubes and tips.
- We recommend maintaining sterile technique whenever possible. However, Forager-1 grows fast and in media with higher salt (and typically antibiotic) than most contaminants tolerate. In settings with sufficient cleanliness and air quality, you may find sterile practices are less necessary.
- For same-day workflows, culture Forager-1 at 37°C.
- For overnight workflows, culture Forager-1 at 30°C or room temp.
- Important! Do not store Forager-1 in a refrigerator! Store plates at room temperature for up to 3-5 days, and prepare 15% glycerol stocks for long term storage at -80°C.
Protocols
Transforming Forager-1 is as simple as adding DNA, mixing, and incubating. Transformed cells can be spread directly onto solid media with selection* to isolate colonies, or used to inoculate a liquid culture for a quick plasmid prep (if clonality is not required or already established).
*See Recovery Note Below
Standard Cloning Protocol
- Prepare LBv2 agar plates by adding 50 ml of 20x v2 Media Salts per 1 L
of molten LB agar with appropriate antibiotic
→ 20x v2 Media Salts may be top-spread on previously made LB plates, but may require significant drying time - Thaw an aliquot of Forager-1 at room temperature (~10 minutes)
- Pre-warm LBv2/selection plates to 37°C
- Add plasmid DNA and mix well (by flicking or vortexing tubes)
- Incubate for 45 minutes – 1 hour at 37°C
→ Incubation can be reduced to as little as 15 minutes, but transformation efficiency is reduced - Spread the transformation on pre-warmed LBv2 plates with appropriate antibiotic selection and dry completely*
→ Spread immediately after pipetting for best results - Incubate plates at 37°C for up to 12 hours (colonies appear in 6-8 hours), or overnight at 30°C or room temperature
- Screen colonies and prepare plasmid according to your preferred methods, and make glycerol stocks within 3 days
→ Do not store plates below room temperature! Plates can be stored at room temperature for up to 3 days.
*Recovery Note: Since doubling of transformed cells could inflate efficiency, we do not recommend “recovering” in rich media before plating. Unlike conventional competent cells, Forager-1 is capable of expressing selection markers during the transformation. Higher apparent efficiencies are possible by including a recovery, but our recommended antibiotic concentrations are optimized for immediate plating. Include a recovery phase at your own discretion and risk.
Quick Plasmid Propagation Protocol
To quickly propagate a clonal plasmid (e.g. to recover or generate more of a previously isolated plasmid):
- To quickly propagate a clonal plasmid (e.g. to recover or generate more of a previously isolated plasmid):Thaw an aliquot of Forager-1 at room temperature (~10 minutes)
- Add 50 ng plasmid DNA and mix well (by flicking tubes or vortexing)
- Incubate for 30 minutes at 37°C
- Add 1.0 ml of liquid LBv2, transfer to a culture tube and incubate at 37°C with shaking for 30 minutes
- Add appropriate antibiotic to the culture from a concentrated stock solution and resume incubation at 37°C with shaking for >6 hours → Follow any culture recommendations from your plasmid preparation kit manufacturer (if available) for optimal yield
- Isolate plasmid with any standard plasmid mini-prep kit
Media recipes
Forager-1 grows well in many published media recipes optimized for Vibrio natriegens. We recommend LBv2, a formulation of LB Miller with added salts. A stock solution of 20x v2 Media Salts is included to easily prepare LBv2 by simply adding 1/20th volume (i.e. 0.25 ml : 5 ml) to liquid LB or molten LB agar. Richer media formulations such as TBv2 may improve plasmid yield, but optimal antibiotic concentrations and growth conditions may vary.
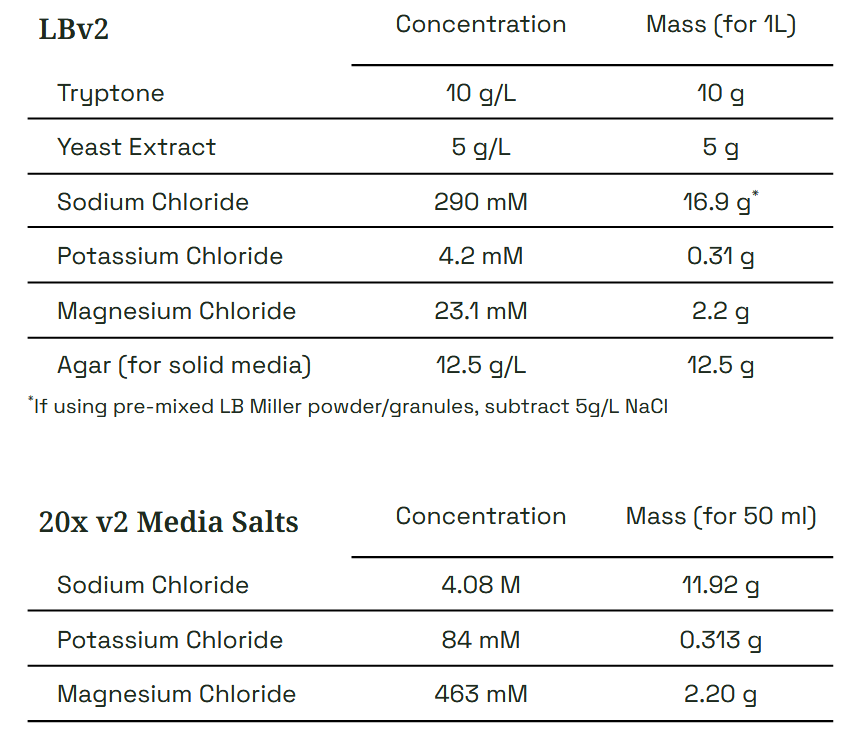
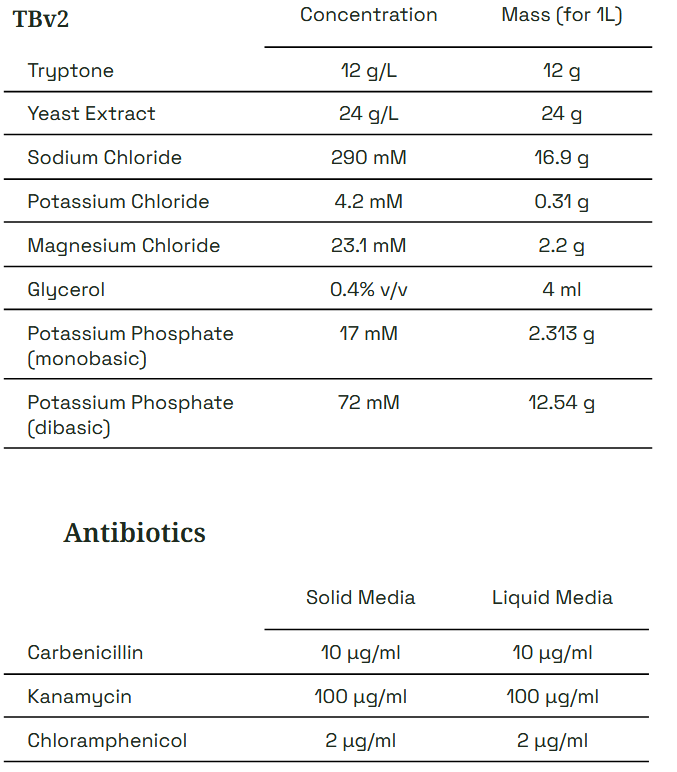
*Other antibiotics and concentrations have been reported for V. natriegens, but we find these work best for Forager-1 in Forager-Fast protocols. Use other selection conditions at your own discretion and risk.
Technical Specifications

Benchmarking Performance
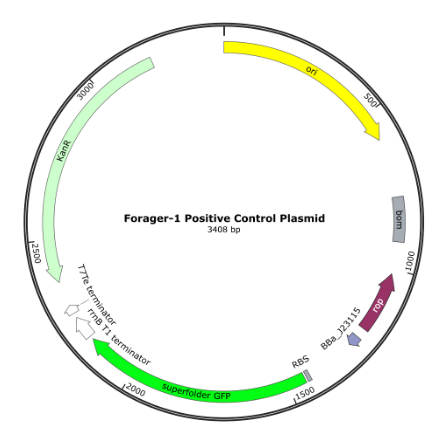
Control plasmid
The Positive Control Plasmid has a Kanamycin selection marker (aminoglycoside phosphotransferase), a high-copy-number origin of replication (pUC), and a fluorescent GFP marker. The full plasmid sequence is available on our website at the product’s page.
Measuring Transformation Efficiency
To measure the transformation efficiency, transform 1 – 100 ng of control plasmid, and prepare a 1:100 dilution of the transformation in LBv2. Spot/spread 50 μl of both the undiluted and diluted transformation on separate 2-3 cm2 areas of an LBv2 agar plate with Kanamycin selection at 100 μg/ml. Allow spots to dry completely and incubate at 37°C for 6 hours or 20-30°C overnight. Count the number of colonies, and calculate the transformation efficiency:
![]()
Measuring Transformation Frequency
To measure the transformation frequency, while measuring efficiency make a 1:1×107 dilution of the transformation in LBv2 and spread 50 μl on LBv2 agar plates with no antibiotic, and calculate the transformation frequency:
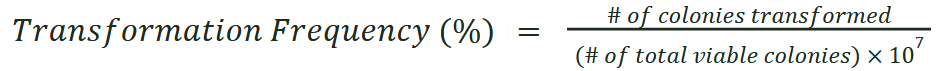
The positive control plasmid contains a GFP reporter to easily verify true positive transformants. Image or view plates under blue light illumination with appropriate filtering (488 nm ex./509 nm em. or orange screen), and prepare fresh plates if an unsatisfactory number of colonies are non-fluorescent. Plates should be incubated at 37°C for at least 2 hours before imaging or viewing for maximum fluorescence.
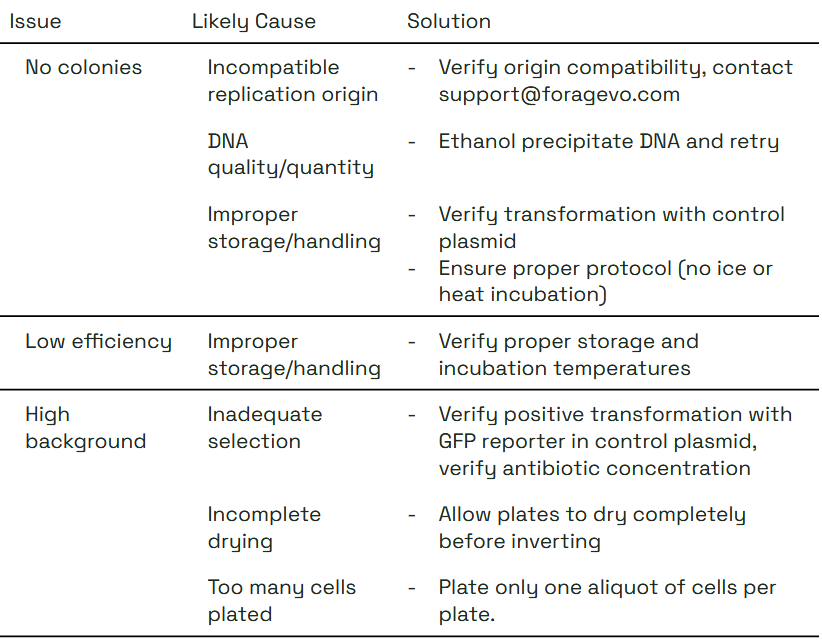
Troubleshooting
Ordering & Support
Order at www.forageevo.com/products. Academic and educational discounts available by request, email customerservice@forageevo.com. For technical support, email support@forageevo.com. For opportunities to help us develop the next generation of Forager-1 and earn rewards, check the product page at www.forageevo.com/products/ffpsk.
Safety & Regulatory
All products are for R&D use only, and not for use on humans or animals. In general V. natriegens is not known to be a human pathogen and is typically classified as BSL1. All kit components are non-toxic. MSDSs are available on our website at www.forageevo.com/protocols and on the product’s page. It is your responsibility to verify safe and compliant use of our products. Product specifications may be updated at any time, always reference the latest documentation. See our terms and conditions for details, available at www.forageevo.com/terms-conditions.
Limited Use Statement
The Forager-Fast Plasmid Subcloning Kit is only to be used for direct transformation and propagation of plasmid DNA. To inquire about other uses and request expanded rights, email us at customerservice@forageevo.com. See our terms and conditions for details.
Resources and References
The body of literature and community generated resources for working with V. natriegens is growing. We recommend the following for learning about V. natriegens as a host organism and for additional context when incorporating Forager-1 into your workflows for the first time. These are not owned, authored, or in any way affiliated with Forage Evolution Inc., always reference product information for Forager-1 specific recommendations and contact us with any questions.
Online Resources
- https://bacdive.dsmz.de/strain/17265
- https://microbewiki.kenyon.edu/index.php/Vibrio_natriegens
- https://barricklab.org/twiki/bin/view/Lab/ProtocolsWorkingWithVibrioNatriegens
Literature References
- Specht DA et. al., Efficient natural plasmid transformation of Vibrio natriegens enables zero-capital molecular biology, PNAS Nexus, 2024
- Weinstock MT et. al, 2016, Vibrio natriegens as a fast-growing host for molecular biology, Nature Methods, 2016
- Hoffart E et. al., High Substrate Uptake Rates Empower Vibrio natriegens as Production Host for Industrial Biotechnology, Applied and Environmental Microbiology, 2017
